Rank the Given Compounds Based on Their Relative Acidities
For the following reaction indicate which reactant is. Rank the following Bronsted acids from strongest to weakest.
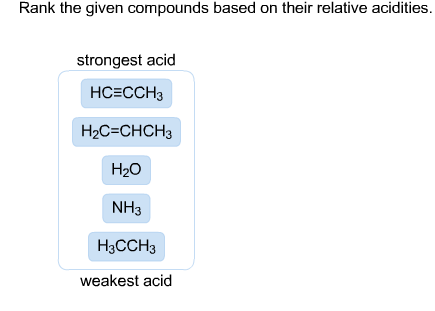
Solved Rank The Given Compounds Based On Their Relative Chegg Com
Two acid-base reaction are given below.
. I couldnt find NH3 in that list but it is 925. Rank the given compounds based on their relative Bronsted aciditiesStrongest Bronsted acid to weakest Bronsted acidH - SHH - FH - IH - CH3H - NH2 - 12122754. H-SH H-CH3 H-NH2 H-F H-I.
Strongest Bronsted acid to weakest Bronsted acid. Rank the given compounds based on their relative acidities. I know that the acidity decreases with an increase in pKabut when I looked up pKa values and tried to use that for finding acidity it did not work.
H - SH H - F H - I H - CH3 H - NH2 A stock has a beta of 128 the expected return on the market is 12 percent and the risk-free. H-I H-F H-SH H-NH2 H-CH3. Strongest acid H2C-CHCH3 HCECH 3CNH2 H3CCH3 H2O weakest acid.
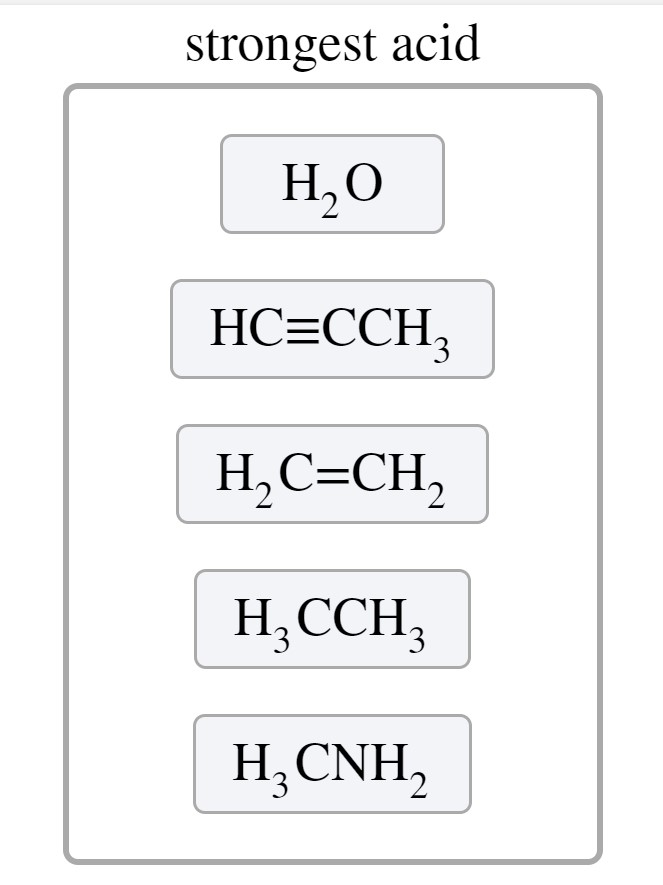
H2O HCtriplebondCCH3 H3CNH2 H2CCHCH3 H3CCH3. Rank the given compounds based on their relative acidities. Rank the given compounds based on their relative acidities strongest acid weakest acid H2CCH2 CH4 H20 H3CNH2 HCCH Identify the Bronsted-Lowry acid and base in the following reactions.
Here is a table that lists the pKa for organic and inorganic compounds. Rank the given compounds based on their relative Brønsted acidities. Rank the given compounds based on their relative Bronsted acidities.
H-CH3 H-OH H-Cl H-F H-NH2. Rank the given compounds based on their relative Bronsted acidities. NH2 CH3-CH2-CH2-CH-CH2-CH3 2.
That means HF is a stronger acid than H2O. Rank the given compounds based on their relative acidities. Of the three reaction arrows presented in each reaction select the arrow via the multiple choice circle before it that best indicates the acid base equilibrium position.
Rank the given compounds based on their relative acidities. Strongest acid HF HCCH HCCH NH HCCH weakest acid. Strongest Bronsted acid weakest Bronsted acid H - CH_3 H - NH_2 H - SH H - F H - I.
Rank the given compounds based on their relative Bronsted acidities. Rank the given compounds based on their relative acidities. HC triple bonded to CH.
Solution for Rank the given compounds based on their relative acidities. Rank the given compounds based on their relative Brønsted acidities. Osp hybridized carbon N sp² hybridized carbon sp hybridized carbon H2O CHCH NH CH2 CHCH CH.
Strongest Bronsted acid to weakest Bronsted acid. I thought it correlated with whichever element was more electronegative was also more acidic. The compounds are ranked from highest to lowest based on their relative acidities as follows.
Strongest acid weakest acid Answer Bank HCNH HCCCH HF HCCH2 CH. Rank the given compounds based on their relative acidities. An explanation would be much appreciated.
Strongest acid H3CNH2 CH4 H2C-CHC3 HCECCH3 HF weakest acid Incorrect Amines. Drawing Lewis structure 1. Be sure you look in the water column and not the.
For example H2O is 157 and HF is 317. H - SH H - F H - I H - CH3 H - NH2. Rank from strongest to weakest acid.
I tried to put them in order of acidity based on their pKa values but it did not work.

Solved Rank The Given Compounds Based On Their Relative Chegg Com
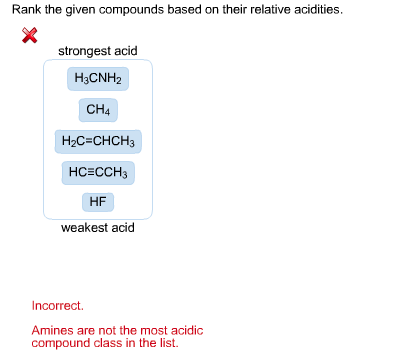
Solved Rank The Given Compounds Based On Their Relative Chegg Com

Solved Rank The Given Compounds Based On Their Relative Chegg Com

Comments
Post a Comment